Atoms & Radioactivity
- Jack Daly

- Feb 18, 2019
- 5 min read
Updated: Feb 21, 2019
Inside An Atom
To understand Radioactivity we must first understand what an atom is and what it is made of. An Atom is made up of 3 different particles. These are known as: Protons, Neutrons and Electrons. On you left we can see a diagram of how these particles create an Atom. Here you can see we have 2 sections: the Nucleus, this contains both the protons and neutrons; the outer shells which are the surrounding rings that contain electrons. On the right, however, you can see a table that shows some key information as to there charges and mass. These are super important and i will explain why later on.
The Atom
Every element on the periodic table has a format like this, where: X is the Element Symbol; A is the Mass number and Z is the Atomic Number. So what is the Mass and Atomic number?
The Mass Number is referencing to the amount of mass in the atom. Now, we know that electrons don't have a mass (its very small and we don't need to take this to account in GCSE) therefore the Mass number is the number of neutrons + the number of protons or the relative atomic mass (just anther way of saying Mass number - Highly Recommended) of the nucleus.
The Atomic number is simply the number of Protons in the Atom.
So we can tell from the numbers given the amount of protons, and if we need to find the amount of neutrons
we simply minus the Atomic number from the Mass number. But how do we get the amount of electrons? Well, in an atom, the number of protons is in direct proportion or equal to the number of electrons. This means that the atomic number is also equal to the amount of electrons.
----

Why are they the same? Well Protons are positively charged while electronics, negatively charged. This means that there can be any number of neutrons (isotopes) but as long as the electrons and protons are equal, it will be an atom. As soon as the magnitude of electrons change (amount of electrons) the atom becomes an ion due to the changed charge.
Isotopes
I mentioned Isotopes in the above description, so what are they? An isotopes is an atom that has the same number of protons and electrons however it varies in the amount of neutrons it has. This is due to the number of protons being used to identify that element. An example is hydrogen. Here we can see 3 Hydrogen Isotopes. We have Hydrogen, which is the element we all know and love; Deuterium, which is actually used in fusion (like Tritium). The different between Hydrogen and Deuterium is that Deuterium has 1 neutron. All this does it change the relative atomic mass of that isotope. And finally, Tritium, which has 2 neutrons.
But how does this effect the physical properties of such element. Well, it affects the mass but anther - less obvious - effect is the increase in instability. The increase in stability is due to the gain or loss in neutrons. This is due to the nuclear forces that help keep the nuclear together against the repelling electrons (keep in mind the nucleus is positively charged while the shells are negatively charged - opposite forces repel). An unstable Nucleus will slowly decay over time, as it decays it gives out energy and potentially Alpha and Beta Particles. This is called ionizing Radiation. Ionizing radiation causes atoms to gain or loose charge meaning they turn into an ion. Unstable nuclei decay at random.

There are 3 basic types of ionizing radiation. there are alpha, beta and gamma rays
Alpha Particle
An alpha particle is made up of 2 protons and 2 neutrons (this intern makes it have a high mass, sound familiar? This is also the same as a Helium Nuclei and has a charge of 2 + due to the two positive protons.
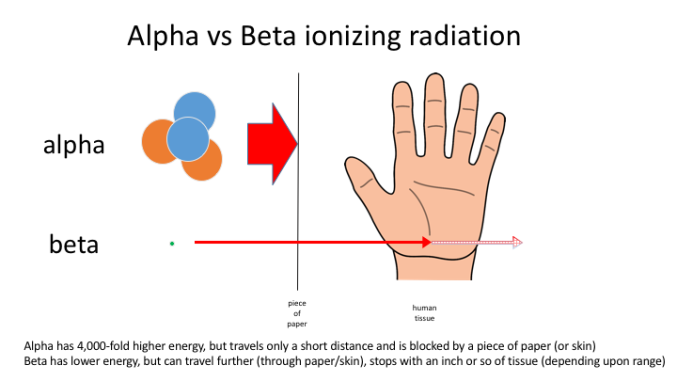
Alpha particles have a small range of ionizing radiation, this means that the distance it travels through matter is short. Alpha particles can travel a short distance through air and only a couple of mm through paper. This is due to the Alpha particle interacting with atoms along its way, giving up its energy quickly creating ions.
Beta Particle
A Beta particle is a high speed electron that is ejected from an unstable atom. The stability of an atom is dependent on the ratio between protons and neutrons. The end goal of radioactive decay is to balance the number of protons an neutrons. A Beta Particle is the result of a neutron splitting into a proton and a electron in the nuclei (due to the energy and positive charge, the negatively charged electron is flung out).
Due to the lower mass that a Beta particle has, they interactive with less atoms therefore increasing the range a Beta Particle can travel. They can also travel through paper however a few milometers of Aluminum is enough to stop the Beta Particle.
Gamma Rays

Gamma rays are waves with very short wave lengths. This means it has no charge nor mass, allowing it to travel through nearly all materials (lead, for example, is good at stopping gamma waves). They are weekly ionizing because they are electrically neutral and ionization is caused by charged particles.
Neutron Radiation

Neutrons are emitted by radioactive materiel. They are not electrically charged therefore not directly causing ionization. They are, however, absorbed by other atoms and can cause them to become radioactive, this is the only form of radiation that causes other atoms to become radioactive and release ionizing radiation.

Decay
Alpha Decay

The General Formula for Alpha Decay is:
Here you can see how each side for A and Z must be equal
Keep in mind energy is also produced in this reaction.
An example of this is radium 222 decays, releasing an alpha particle, turning into radon.
Beta Decay

Here we can see we have two types of Beta Decay. Beta Plus and Minus. This is due to there being two types of beta particles being released. A positive Beta particle will be released if a proton decays into a neutron.
Here, in the formulas, we can see that the number of neutrons decreases by one, due to it being decayed, and the number of protons increasing by one, in Beta minus Decay. However in Beta positive Decay we see the opposite.
The general formula for Beta minus is:
A A 0
Y -> X + e
Z Z + 1 - 1
Neutron Decay

Here we can see we have two types of Beta Decay. Beta Plus and Minus. This is due to there being two types of beta particles being released. A positive Beta particle will be released if a proton decays into a neutron.
Here, in the formulas, we can see that the number of neutrons decreases by one, due to it being decayed, and the number of protons increasing by one, in Beta minus Decay. However in Beta positive Decay we see the opposite.
The general formula for Beta minus is:
A A 0
Y -> X + e
Z Z + 1 - 1
Gamma Decay
Due to a Gamma ray being pure energy, as it is electromagnet radiation, there is no change to neither the mass or atomic numbers. Gamma rays have no mass or charge.
Remember To comment if your stuck with anything and feel free to answer any questions below. Keep learning !















oh wow your'e good at chemistry. From this it looks like you are set 1. you are set 1 Right?